Biological Analyses Laboratory
Medical device testing
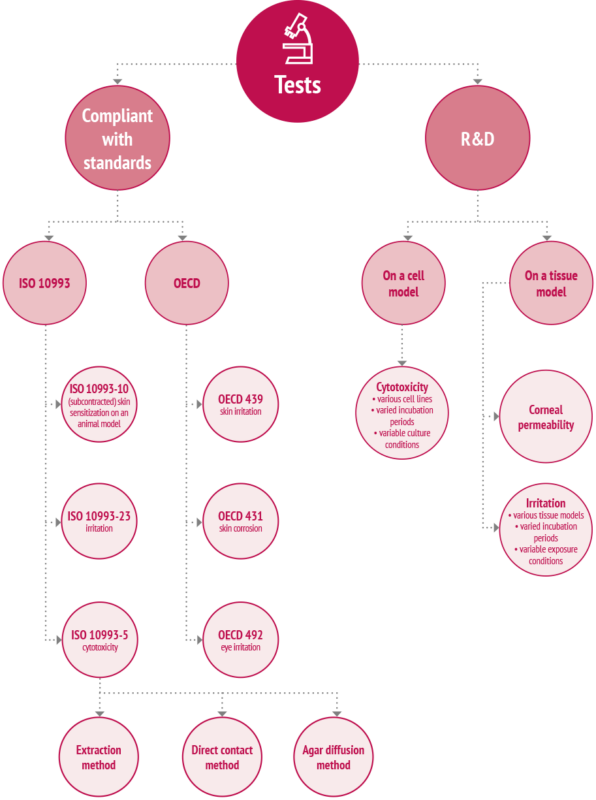
Quantitative cytotoxicity in vitro, using neutral red uptake (NRU) assay as per ISO 10993-5
The cytotoxicity test is aimed at determining general toxicity of medical devices and materials. The assay includes extraction of medical devices and materials in a culture medium (as per ISO 10993-12) and then exposing murine fibroblasts (Balb3t3) to the extract. Evaluation of cytotoxic effect is performed quantitatively, using the neutral red uptake (NRU) assay. It allows quantitative determination of the amount of captured staining material after exposing the cells to the tested substance accumulated in the lysosomes of living cells.
The test is performed on all medical devices that have contact with the patient and on all raw materials and devices that are submitted to validation of cleaning or remaining production.
Skin irritation test using reconstructed human epidermis (RHE) as per ISO 10993-23
In vitro skin irritation test is used to predict acute skin irritation by medical devices. The assay includes extraction of medical devices and materials in physiological saline solution and sesame oil (as per ISO 10993-12) and then exposing reconstructed human epidermis – RHE – to the extract over a set time. The irritation effect is evidenced by increased cytotoxicity of the extracts to RHE, which is measured quantitatively using MTT test. The MTT test allows measurement of mitochondrial enzymes activity in the cell, which is proportional to the amount of reduced tetrazole salt, and hence can be a determination of cell viability.
Please note! In JCI, we do not conduct tests of implant (implanted medical devices).
Cell tests
- Cytotoxicity using MTT, NRU assays and IC50 determination
- Cell viability over time
- Oxidative stress test
- Growth factor secretion test
Tests on reconstructed human tissues
- Irritating and/or corrosive effect to skin/eye – an assay using reconstructed human tissues (EpiDerm™, EpiOcular™) in vitro intended as hazard assessment test to predict acute skin or eye irritation by the substance or mixture. The tested sample is applied directly onto the tissue and then the exposition is followed over a set time. The irritation effect is evidenced by increased cytotoxicity, which is measured quantitatively using MTT test. The MTT test allows measurement of mitochondrial enzymes activity in the cell, which is proportional to the amount of reduced tetrazole salt, and hence can be a determination of cell viability.
- Degree of skin/eye irritation – a test using reconstructed human tissues (EpiDerm™, EpiOcular™) in vitro, intended as a screen for risk assessment in order to determine the expected irritating effect based on the results of time to emergence of toxicity. The test is also used to put the tested preparations according to their skin or eye irritation potential.
Skin irritation test using reconstructed human epidermis (RHE) as per OECD 439
The test is intended for all substances registered according to REACH regulation. It allows differentiating and classification of the tested substance as “non-irritating”, which corresponds to “No category” as per GHS, or “irritating”, which corresponds to combined “Category 1” and “Category 2” as per GHS.
Skin corrossion test using reconstructed human epidermis (RHE) as per OECD 431
The test is intended for all substances registered according to REACH regulation. It allows differentiating and classification of the tested substance as “irritating”, which corresponds to “Category 1” as per GHS, or “corrosive”, which corresponds to “Category 2” as per GHS.
Eye irritation test using a model of reconstructed human eye epithelium as per OECD 492
The test is intended for all substances registered according to REACH regulation. It allows differentiating and classification of the tested substance as “non-irritating”, which corresponds to “No category” as per GHS, or “corrosive/irritating”, which corresponds to combined “Category 1” and “Category 2” as per GHS.
Skin irritation degree test
Intended for all substances (pure substances, mixtures, final preparations) to check and determine their potential or degree of skin irritation. It can be applied especially for new cosmetics or personal hygiene products, or after a change in formulation, and allows avoiding test subjects on probands, hence limiting the risk of their exposition to irritants.
Eye irritation degree test
Intended for all substances (pure substances, mixtures, final preparations) to check and determine their potential or degree of eye irritation. Allows assigning the tested sample one of 4 categories of irritation degree, as well as can be applied for substances, for which the standard Draize test is not sensitive, especially for cosmetics intended to be used in the eye area.
Permeability testing
A test consisting in checking the presence of the test sample in the receiving fluid using Franz chambers after passing through the model of a full-thickness reconstructed human epidermis. Test carried out in cooperation with the Laboratory of Chromatography and Mass Spectrometry.
PCR, qPCR, RT-PCR
The Polymerase Chain Reaction allows multiplication of specific DNA fragments in vitro. Real Time-PCR is a quantitative PCR reaction that allows simultaneous multiplication of chosen DNA fragments and monitoring of products formed during subsequent cycles. The Jagiellonian Center of Innovation offers services within the scope of PCR and Real Time PCR optimization using non-specific SYBR Green method or specific method using complementary oligonucleotide probes based on fluorescence resonance energy transfer (FRET) between the donor and quenching molecule. Additionally, we offer RT-PCR (reverse transcription PCR), where RNA is used as a matrix.
The PCR technique has many applications in genome research, characterization of gene expression, gene cloning, and clinical diagnostics.
Team
Head of the Biological Analyses Laboratory
Katarzyna Pocheć
Graduated from biotechnology at the Pharmaceutical Department of the Silesian University of Medicine in Sosnowiec and post-graduate studies. Qualified in medicinal product and device quality control at the Pharmaceutical Deportment of Collegium Medicum in Cracow. In her diploma thesis, she dealt with differentiation of mesenchymal stem cells originating from Wharton’s jelly into cartilage cells. Her professional work started in the Laboratory of Bone Marrow Engineering and Cell Bank in Katowice, where she was deputy of the Responsible Person. In 2017 she joined the Jagiellonian Centre for Innovation to develop the Laboratory of Tissues and Cells in GMP standard. Currently, she is the head of the Biological Analyses Laboratory, where she is responsible for designing and performing analyses.
For a quote, please contact:
Agnieszka Serwinowska
Mobile: +48 797 611 171
E-mail: agnieszka.serwinowska@jci.pl







