JCI Clinical Trials Center
The JCI Clinical Trials Center is a specialized research unit that conducts clinical trials and runs research and development projects in the field of medicine and related sciences.
JCI Clinical Trials Center boasts such facilities as:
- 550 sq m of state-of-the-art bespoke space
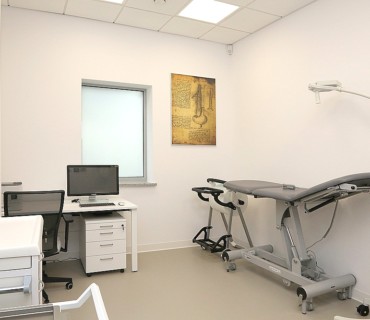
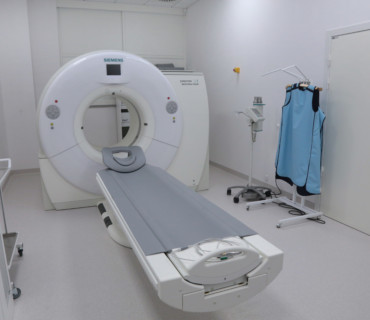
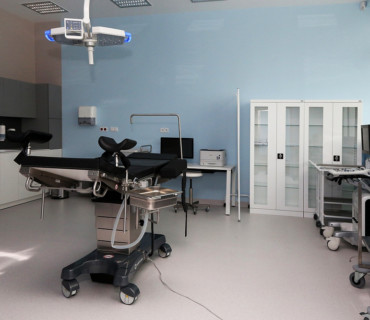
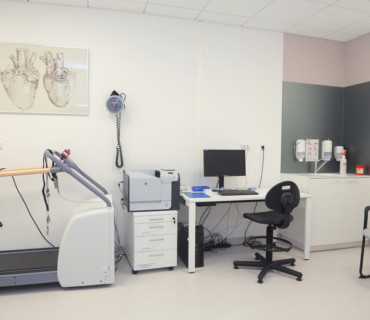
- latest generation diagnostic equipment (such as an MR scanner, CT scanner, ultrasound, densitometer)
- a modern treatment unit with ultrasound systems and a patient bedroom
as well as highly-qualified clinical and research personnel.
The JCI Clinical Trials Center conducts and oversees clinical trials in all medical specialties (phases I – IV), according to sponsors’ instructions.
JCI Clinical Trials Center’s team comprises world-class specialists with many years of experience in conducting clinical trials, research and development activities as well as health care. Specifically, the research and clinical personnel includes, amongst others, doctors specializing in various medical fields, clinical trial nurses, clinical research coordinators, medical physicists, electroradiology technicians and medical secretaries.
The research personnel have comprehensive experience in conducting clinical trials. The team have set for themselves such priorities as utmost consideration for trial participants’ safety, high standards of recruitment as well as high quality, reliable data for the sponsor. All the research personnel regularly attend good clinical practice training.
For more information please visit our website at www.cbkjci.pl
Centrum Badań Klinicznych JCI
Life Science Park, building C
ul. Bobrzyńskiego 14
30 – 348 Kraków
Telephone: +48 12 340 24 02
Mobile: +48 512 047 678
E-mail: cbk@jci.pl