Design and documentation
Jagiellonian Center of Innovation together with partners operating in the healthcare market offers a comprehensive service for entities interested in conducting biomedical experiments attended by participants (healthy and patient). This service includes design, implementation and supervision of:
- research experiments
- clinical trials
- observational studies
The areas covered by the comprehensive service are: medical devices/medical equipment, incl. software, IVD devices, quasi-pharmaceutical medical devices, healthcare applications, well-being solutions, medicinal products.
Implementation of projects with the participation of volunteers (healthy and/or patient) will take place on the basis of the infrastructure of the JCI Clinical Trials Center and in selected partner centers. The stage related to the preparation of project documentation will be implemented in cooperation with independent entities with many years of experience in the scope of:
- design
- management
- monitoring
- and regulators in the area of clinical trials.
Project evaluation process:
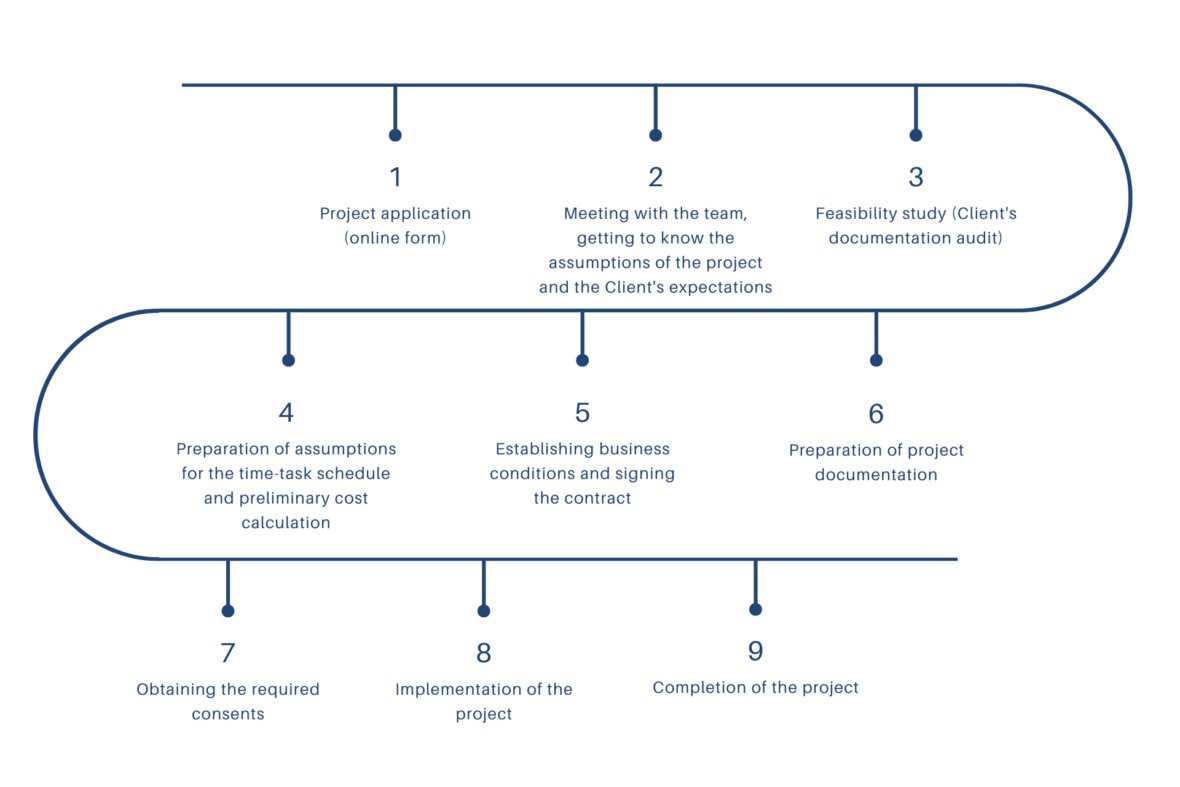
The comprehensive service includes the following activities:
- Preparation of documentation for a research experiment or clinical trial, such as investigator’s brochure, clinical trial protocol and obtaining approvals from the Bioethical Commission and the Office for Registration of Medicinal Products, Medical Devices and Biocides, including:
- preparation of the patient’s informed consent
- preparation of patient record cards
- preparation of a clinical observation card (CRF, eCRF)
- Implementation of the project at the CBK JCI and AWF premises
- Project management, recruitment plan, monitoring plan
Also, as part of additional support services for projects in the medical devices area, we offer:
- development of technical documentation in accordance with the MDR annex II + III
- production of substance-based medical devices and cosmetics, if applicable
- assistance in planning and coordinating laboratory tests – we have 6 laboratories equipped with analytical instruments more information here >>>